**PLEASE USE MATLAB 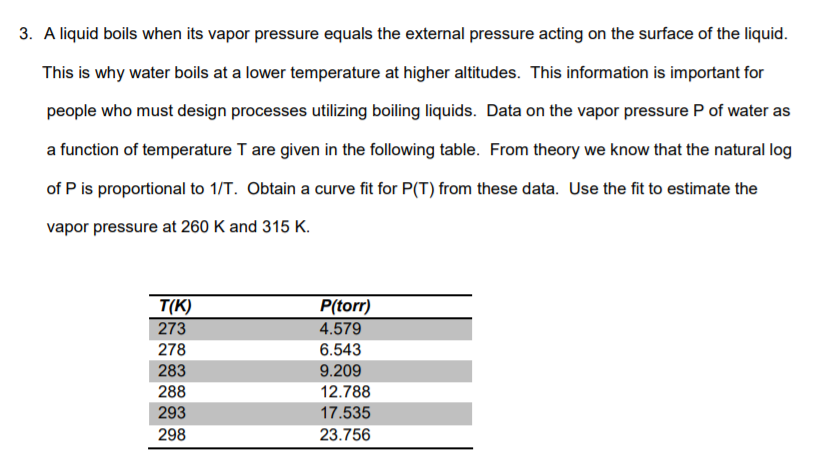
3. A liquid boils when its vapor pressure equals the external pressure acting on the surface of the liquid This is why water boils at a lower temperature at higher altitudes. This information is important for people who must design processes utilizing boiling liquids. Data on the vapor pressure P of water as a function of temperature T are given in the following table. From theory we know that the natural
OR
OR
