do a and c
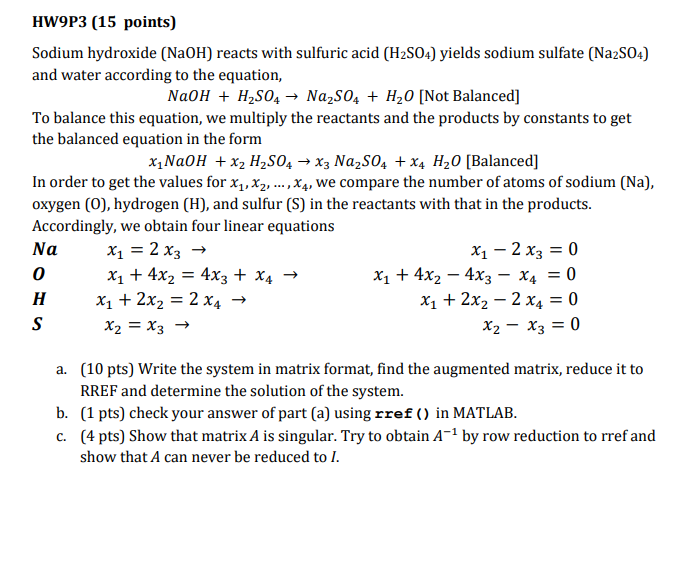
HW9P3 (15 points) Sodium hydroxide (NaOH) reacts with sulfuric acid (H2S04) yields sodium sulfate (Na2S04) and water according to the equation, NaOH + H2SO4→ Na2SO4 + H20 [Not Balanced] To balance this equation, we multiply the reactants and the products by constants to get the balanced equation in the form x1NaOH x2 H2S04x3 Na2S04 + x4 H20 Balanced] In order to get the values for x,x2, …, x4, we compare the number of atoms of sodium
OR
OR
