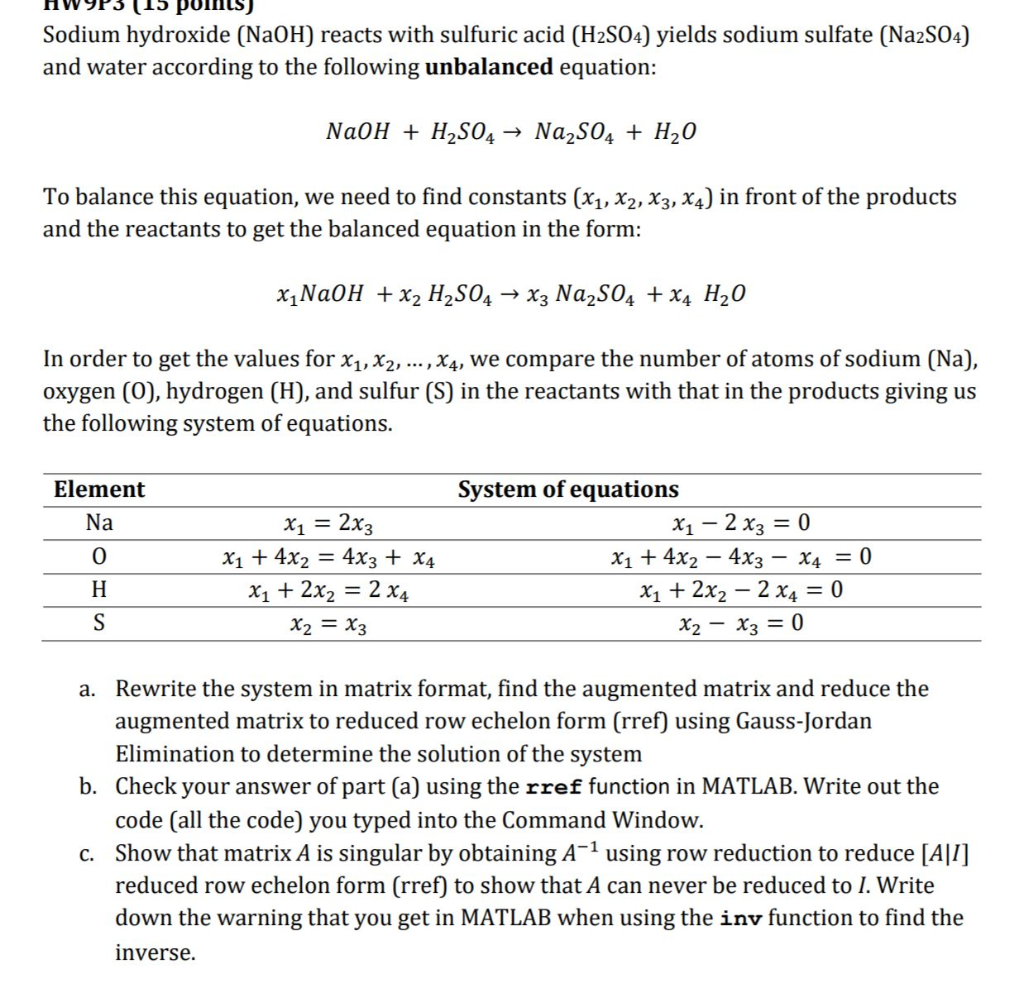
Sodium hydroxide (NaOH) reacts with sulfuric acid (H2S04) yields sodium sulfate (Na2S04) and water according to the following unbalanced equation: NaOH + H2S04-> Na2SO4 H20 To balance this equation, we need to find constants (x1, x2, x3, x4) in front of the products and the reactants to get the balanced equation in the form: In order to get the values for xj x2, …, x4, we compare the number of atoms of sodium (Na), oxygen (O0), hydrogen (H), and
OR
OR
